Abstract
Background: Clinical studies have demonstrated that ≈ 50% of pts with CML-CP in sustained DMR on long-term tyrosine kinase inhibitor (TKI) therapy can stop treatment and achieve TFR. ENESTfreedom and ENESTop are investigating TFR in pts with sustained DMR on frontline or second-line nilotinib, respectively. Among pts who entered the TFR phase of ENESTfreedom or ENESTop, 98/190 (51.6%) and 73/126 (57.9%), respectively, remained in TFR at 48 wk (primary endpoint); 93 (48.9%) and 67 (53.2%) remained in TFR at 96 wk. As an exploratory objective, these studies are investigating the potential to achieve TFR in pts initially deemed ineligible due to unstable DMR but who achieved stable DMR with additional nilotinib treatment.
Methods: Both studies enrolled pts with CML-CP and either ≥ 2 y of frontline nilotinib therapy (ENESTfreedom) or ≥ 3 y of total TKI therapy, including > 4 wk of imatinib followed by ≥ 2 y of nilotinib (ENESTop). Achievement of MR4.5 (BCR-ABL1 ≤ 0.0032% on the International Scale [IS]) with nilotinib prior to enrollment was required for both studies. Upon enrollment, pts entered a 1-y consolidation phase; those not maintaining stable DMR throughout the consolidation phase were ineligible to enter the main TFR phase and instead entered a 1-y nilotinib continuation phase upon completion of the consolidation phase. Pts who maintained stable DMR in the continuation phase stopped treatment and entered the TFR-2 phase. Pts with loss of major molecular response (MMR [ BCR-ABL1IS ≤ 0.1%]; in either study) or confirmed loss of MR4 (BCR-ABL1IS ≤ 0.01%; ENESTop only) during the TFR-2 phase reinitiated nilotinib and entered the reinitiation-2 phase. Results presented here are based on a data cut-off of October 31, 2016, for ENESTfreedom and November 7, 2016, for ENESTop.
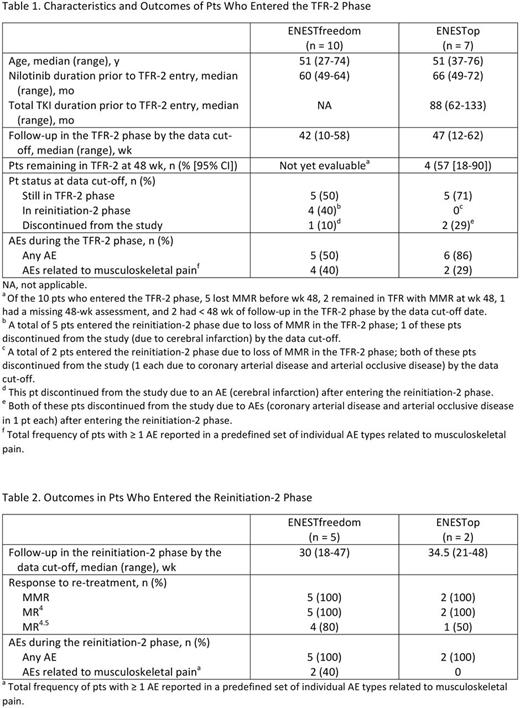
Results: Among 215 pts who entered the consolidation phase of ENESTfreedom, 13 did not maintain stable DMR in the consolidation phase and entered the nilotinib continuation phase; 10 of these 13 maintained stable DMR in the continuation phase and entered the TFR-2 phase. In ENESTop, among 163 pts who entered the consolidation phase, 26 did not maintain stable DMR in the consolidation phase and entered the continuation phase; 7 of these 26 entered the TFR-2 phase. Characteristics of pts in the TFR-2 phase of each study are shown in Table 1.
At the data cut-off for this analysis, 5 of 10 (50%) and 5 of 7 (71%) pts in ENESTfreedom and ENESTop, respectively, remained in the TFR-2 phase; the other 5 and 2, respectively, experienced molecular relapse and entered the reinitiation-2 phase by the data cut-off. Among pts who entered the TFR-2 phase, 1 of 10 in ENESTfreedom and 2 of 7 in ENESTop discontinued from the study by the data cut-off, all due to adverse events (AEs) after reinitiating treatment (ENESTfreedom, 1 due to cerebral infarction; ENESTop, 1 each due to coronary arterial disease and arterial occlusive disease). In ENESTop, 4 of 7 pts (57%) who entered the TFR-2 phase remained in TFR after 48 wk, and 1 had < 48 wk of follow-up by the data cut-off; in ENESTfreedom, the 48-wk TFR rate was not yet evaluable at the data cut-off. In ENESTfreedom, among 5 pts who entered the reinitiation-2 phase, 4 (80%) regained MR4.5 (the pt without MR4.5 discontinued from the study after achieving MR4). In ENESTop, the 2 pts who entered the reinitiation-2 phase regained MR4 and MR4.5, respectively, prior to discontinuing from the study (Table 2). No cardiovascular events (CVEs) were reported in the TFR-2 phase of either study; in the reinitiation-2 phase, a total of 3 pts across both studies had AEs related to CVEs, all of which led to treatment discontinuation.
Conclusion: Among pts in the TFR-2 phase of ENESTfreedom or ENESTop, the proportion able to remain off treatment without molecular relapse appears to be similar to that among pts who entered the main TFR phase of each study. Thus, although stable DMR is an absolute prerequisite for attempting TFR, these data suggest that some pts with unstable DMR are able to achieve the level of response needed to stop treatment successfully with additional nilotinib therapy. These results must be interpreted with caution due to the small sample size and short follow-up.
Hughes: Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hochhaus: ARIAD: Research Funding; Incyte: Research Funding; Novartis: Research Funding; BMS: Research Funding; MSD: Research Funding; Pfizer: Research Funding. Kim: Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Il-Yang: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Mahon: Novartis: Research Funding, Speakers Bureau; Pfizer: Speakers Bureau; BMS: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau. Giles: Novartis: Consultancy, Research Funding. Radich: Ariad: Consultancy; Gilliad: Consultancy; Amgen: Consultancy; Novartis: Consultancy, Other: lab contracts for bid and service assays; BMS: Consultancy; Pfizer: Consultancy. Gopalakrishna: Novartis: Employment, Equity Ownership. Deng: Novartis Pharmaceuticals Corporation: Employment. Fellague-Chebra: Novartis: Employment. Acharya: Novartis: Employment. Saglio: Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Ariad: Consultancy, Honoraria; BMS: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal